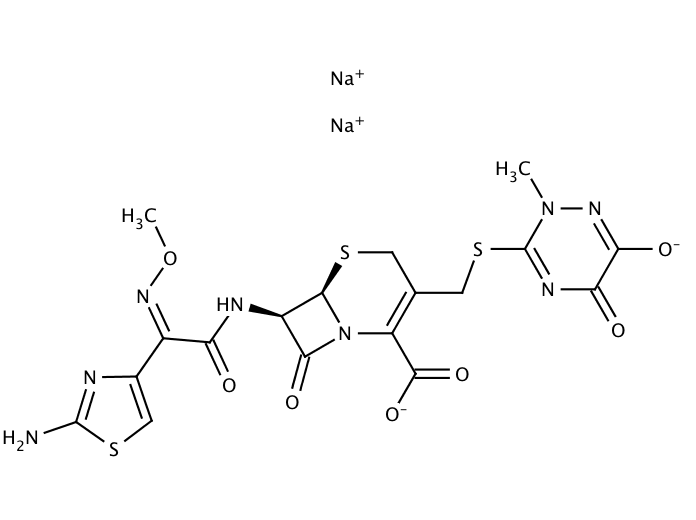
Ceftriaxone sodium salt
CAS: 104376-79-6
Molecular Formula: C18H16N8Na2O7S3 · 3.5H2O
Molecular Weight: 661.6
Product Detail
| Product Code | 10212773 | ||||
| Product Name | Ceftriaxone sodium salt | ||||
| Categories | Antibiotics, Bioactive Small Molecules, Research Organics & Inorganics | ||||
| CAS | 104376-79-6 | ||||
| Molecular Formula | C18H16N8Na2O7S3 · 3.5H2O | ||||
| Molecular Weight | 661.60 | ||||
| Storage Details | 2 to 8 °C | ||||
| Harmonised Tariff Code | 29349990 |
Product Specification
| Grade | USP 43 | ||||
| Water | 8.0 – 11.0 % | ||||
| pH | 6.0 – 8.0 | ||||
| Bacterial endotoxin | Conforms to USP 43 | ||||
| Acetone | ≤ 0.5% | ||||
| Solubility | Clear, colourless to yellow | ||||
| Sterility | Conforms to USP 43 | ||||
| Total Impurities | ≤ 2.5% | ||||
| Crystallinity | Conforms to USP 43 | ||||
| Appearance | White to yellowish-orange crystalline powder | ||||
| Residual Solvents:Methanol | ≤ 0.3% | ||||
| Residual Solvents: Ethanol | ≤ 0.5% | ||||
| Assay (Anhydrous) | 795u g/mg min | ||||
| Identification (IR) | Conforms to USP 43 | ||||
| Any single impurity | ≤ 0.2% | ||||
| Identification c, test for sodium | Conforms to USP 43 | ||||
| Residual Solvents: Triethylamine | ≤ 0.02% |
Safety Information
| Pictograms |
|
||||
| Signal word | Danger | ||||
| Hazard statements |
H315: Causes skin irritation H317: May cause an allergic skin reaction H319: Causes serious eye irritation H334: May cause allergy or asthma symptoms or breathing difficulties if inhaled H335: May cause respiratory irritation |
||||
| Precautionary statements |
P261: Avoid breathing dust/fume/gas/mist/vapours/spray P280: Wear protective gloves/protective clothing/eye protection/face protection P284: Wear respiratory protection P304+340: IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing P305+351+338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present and easy to do, continue rinsing |
